Research Group Experimental Deep brain Stimulation
The Experimental Deep Brain Stimulation working group (Group Leader: Dr Mareike Fauser) is primarily concerned with the effects and mode of action of deep brain stimulation in animal models of Parkinson's disease. Our aim is to better characterise this procedure, which has been available in clinical use for decades, and thereby contribute to the future optimisation of deep brain stimulation in Parkinson's patients.
Parkinson’s disease is one of the most common neurodegenerative diseases with a prevalence of approx. 100 per 100,000 in the age group up to 60 years, although up tp to approx. 3,000 per 100,000 in the over-80s. It classically presents clinically with the triad of (resting) tremor, rigor and bradykinesia. In recent years and decades, however, so-called non-motor symptoms evolved as a major player, particularly in patients' quality of life. Certain non-motor symptoms (e.g. olfactory disorders and REM sleep behaviour disorder) can even occur before the onset of motor symptoms. To date, the available treatment methods for both motor and nonmotor symptoms are exclusively symptomatic in nature and disease modification is not on the horizon. Deep brain stimulation (DBS) in Parkinson's disease patients has so far primarily been used in the middle to late stages of the disease, when drug therapy approaches no longer lead to sufficient symptom relief, but could also have the potential to alleviate non-motor symptoms that occur at an early stage.
In our research group, we are currently investigating the effect of DBS on cellular plasticity, analysing the influence on various dopaminergic systems as well as on adult neurogenesis in an established hemiparkinsonian model. Here we could show that long-term deep brain stimulation leads to an increase of dopaminergic cells in the midbrain as well as to a proliferation of newly generated dopaminergic interneurons in the olfactory bulb. These effects appear to be dependent on the site of stimulation and could only be demonstrated with stimulation in the subthalamic nucleus and not with stimulation in the globus pallidus pars internus (in the rat: nucleus entopeduncularis). Currently, we are particularly interested in the potential link between cellular plasticity and non-motor symptoms of Parkinson's disease, using an alternative alpha-synuclein expressing animal model. In addition, we are interested in the influence of DBS on the expression of trophic factors in certain brain areas. These data are additionally verified by means of in vitro studies in adult neuronal stem cells.
As part of the Collaborative Research Centre ELAINE, we are working on the technical optimisation of preclinical stimulation systems (sub-project C04; funded by the German Research Foundation III/2021-IV/2025) and established a fully implantable stimulation system in experimental animal models (in collaboration with the Institute for Applied Microelectronics and Data Technology, Prof. Dirk Timmermann, Prof. Christian Haubelt). With these, functional influences can be investigated much better than with external stimulators available, which had been available before. In addition and also as part of the consortium, we are investigating the field distribution and potential influences of the electrode material and the stimulation mode on the effects of DBS with colleagues from electrical engineering (Institute of General Electrical Engineering, Chair of Theoretical Electrical Engineering, Prof Ursula van Rienen).
![[Translate to English:] PCR](/fileadmin/_processed_/5/1/csm_PCR_395993ee61.jpg)
Equipment (a selection):
Digital two-arm stereotaxic frame (Stoelting Neuroscience)
Research Cryostat /Cryomicrotome CM3050S (Leica)*
High-resolution fluorescence microscope AxioObserver.Z1 with Apotome (Zeiss)
* co-financed by the European Union from the European Regional Development Fund
Publications
- Fauser M, Payonk JP, Weber H, Statz M, Winter C, Hadar R, Appali R, van Rienen U, Brandt MD, Storch A. Subthalamic nucleus but not entopeduncular nucleus deep brain stimulation enhances neurogenesis in the SVZ-olfactory bulb system of Parkinsonian rats. Front Cell Neurosci. 2024 Apr 30;18:1396780. doi: 10.3389/fncel.2024.1396780.
- Statz M, Schleuter F, Weber H, Kober M, Plocksties F, Timmermann D, Storch A, Fauser M. Subthalamic nucleus deep brain stimulation does not alter growth factor expression in a rat model of stable dopaminergic deficiency. Neurosci Lett. 2023 Sep 25;814:137459. doi: 10.1016/j.neulet.2023.137459.
- Helf C, Kober M, Markert F, Lanto J, Overhoff L, Badstübner-Meeske K, Storch A, Fauser M. Subthalamic nucleus deep brain stimulation induces nigrostriatal dopaminergic plasticity in a stable rat model of Parkinson's disease. Neuroreport. 2023 Jun 7;34(10):506-511. doi: 10.1097/WNR.0000000000001917.
- Fauser M*, Loewenbrück KF*, Rangnick J, Brandt MD, Hermann A, Storch A. Adult Neural Stem Cells from Midbrain Periventricular Regions Show Limited Neurogenic Potential after Transplantation into the Hippocampal Neurogenic Niche. Cells. 2021 Nov 4;10(11):3021. doi: 10.3390/cells10113021.
- Fauser M, Ricken M, Markert F, Weis N, Schmitt O, Gimsa J, Winter C, Badstübner-Meeske K, Storch A. Subthalamic nucleus deep brain stimulation induces sustained neurorestoration in the mesolimbic dopaminergic system in a Parkinson's disease model. Neurobiol Dis. 2021 Aug;156:105404. doi: 10.1016/j.nbd.2021.105404.
- Fauser M, Pan-Montojo F, Richter C, Kahle PJ, Schwarz SC, Schwarz J, Storch A, Hermann A. Chronic-Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis. Cells. 2021 Mar 31;10(4):775. doi: 10.3390/cells10040775.
- Fauser M, Weselek G, Hauptmann C, Markert F, Gerlach M, Hermann A, Storch A. „Catecholaminergic innervation of periventricular neurogenic regions of the developing mouse brain”. In: Front Neuroanat 14 (2020).
- Weselek G, Keiner S, Fauser M, Wagenführ L, Müller J, Kaltschmidt B, Brandt MD, Gerlach M, Redecker C, Hermann A, and Storch A. “Norepinephrine is a negative regulator of the adult periventricular neural stem cell niche”. In: Stem Cells 38.9 (2020), pp. 1188–201.
Effects of deep brain stimulation in the subthalamic nucleus (STN-DBS) on adult neurogenesis in the olfactory bulb (OB) in unilaterally 6-OHDA-lesioned rats.
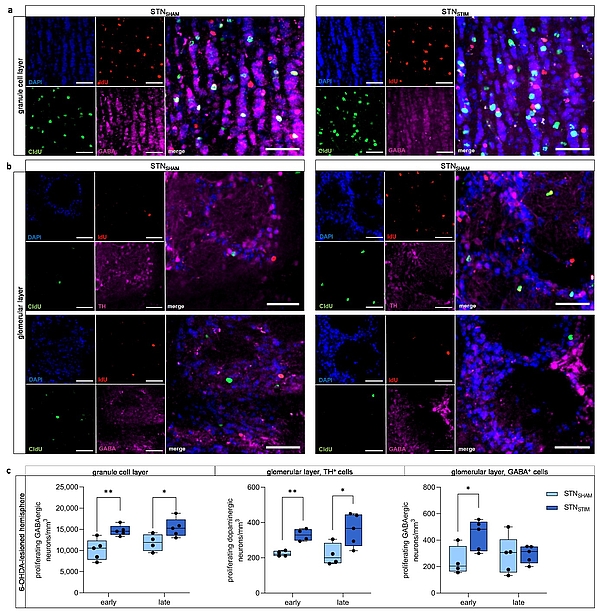
Figure 1: Effects of deep brain stimulation in the subthalamic nucleus (STN-DBS) on adult neurogenesis in the olfactory bulb (OB) in unilaterally 6-OHDA-lesioned rats.
(a, b) Representative immunohistological images of newborn neurons in the OB under STNSHAM and STNSTIM conditions. IdU (red) marks newborn neurons that were generated early (two days) after DBS onset, while CldU (green) labels newborn neurons after three weeks of continuous DBS. GABAergic neurons (pink) are found in both the granule layer (a) and the glomerular layer(b, lower panel), while TH+ dopaminergic neurons (pink) are restricted to the latter (b, lower panel). Cell nuclei were counterstained with DAPI. Scale bar, 50 µm. (c) A significant increase in the number of newborn GABAergic neurons in the granule layer and TH+ dopaminergic neurons in the glomerular layer was observed both early after STN-DBS onset and three weeks later, while GABAergic neurons in the glomerular layer were only significantly increased immediately after STN-DBS onset compared to SHAM stimulation. All results were obtained in 6-OHDA-lesioned hemispheres (published in Fauser et al., Front Cell Neurosci, 2024).

Present Members:
Mareike Fauser, MD (Junior Research Group Leader)
M.Sc. Meike Statz (PhD student)
M.Sc. Hanna Weber (PhD student)
Uta Naumann (veterinary engineer)
Sarah Pfeisinger (master student)
Valeriia Abramenko (IRTG stipend, medical student)
Maximilian Steinigk (Arzt, medical student)
Caroline Ocker (medical student)
Raja Barthel (medical student)
Past members:
Kathrin Badstübner-Meeske, PhD
Francia Molina, PhD
M.Sc. Maria Kober (PhD student)
M.Sc. Frederike Schleuter (master student)
Franziska Backhaus (medical student)
Felix Bernsdorff (medical student)
Charlotte Helf (medical student)
Martin Nüssel (medical student)
Leonie Overhoff (medical student)
Manuel Ricken, MD

![[Translate to English:] Fauser](/fileadmin/_processed_/c/0/csm_Fauser_6f3b6e9470.jpg)
![[Translate to English:] MV](/fileadmin/_processed_/9/b/csm_MV_06acda98c7.jpg)
![[Translate to English:] EU](/fileadmin/_processed_/8/f/csm_EU_ef61ab803c.jpg)