Research Group on Embryonic Neurogenesis
The research group on embryonic neurogenesis (head: Prof. Dr. Alexander Storch) focuses on neuronal plasticity during brain development. The aim of our work is to investigate the influence of oxygen on embryonic neural stem cells and the formation of different brain regions to elucidate the underlying mechanism of oxygen-dependent neurogenesis.
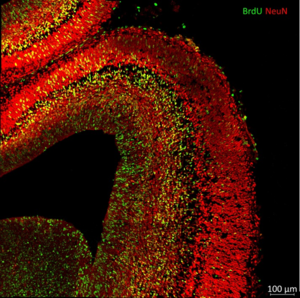
Oxygen is probably the most important gas for our survival and plays a major role in our energy metabolism. However, it also acts as an important signal factor for various cellular processes from the formation of new blood cells to brain development. To regulate these processes, so-called hypoxia inducible factors (HIFs) are produced in our body cells. William G. Kaelin, Peter J. Ratcliffe and Gregg L. Semenza were awarded the Nobel Prize for Medicine in 2019 for the discovery of these HIFs and their underlying mechanism of action. In short, HIF proteins are permanently expressed in our cells, but are immediately degraded under normoxic conditions. A reduced oxygen level, however, slows down the degradation of HIF proteins, so that they in turn can regulate the expression of other nuclear genes.
Since it has long been known that oxygen also influences brain development, it seems likely that the mechanism via HIFs also plays a decisive role in the developing brain. However, how far-reaching this mechanism is and whether it can even be influenced to counteract hypoxic fetal events, for example, is still not well known. Using various in vitro and in vivo methods, we analyze cellular mechanisms in both neuron and stem cell cultures as well as effects on embryonic neuronal plasticity in genetically modified in vivo models in which we can specifically switch off the HIF subunits (HIF1a and HIF2a). In order to simulate different oxygen conditions, we can also perform these experiments in hypoxic or hyperoxic atmosphere.
The current projects are particularly concerned with the perinatal regulation of brain structure after hypoxic and hyperoxic events during brain development.
Publications
Lanto, J., M.M.N. Vehlken, V. Abramenko, A. Storch, and F. Markert, Hyperoxia shows duration-dependent effects on the lengths of cell cycle phases in fetal cortical neural stem cells. Front Cell Dev Biol, 2025.
Weber, H., M. Statz, F. Markert, A. Storch, and M. Fauser, Circadian variations influence anxiety-related behaviour, olfaction, and hedonic response in male Sprague-Dawley rats. Behav Brain Res, 2024.
Walter-Manucharyan, M., M. Martin, J. Pfutzner, F. Markert, G. Rodel, A. Deussen, A. Hermann, and A. Storch, Mitochondrial DNA replication is essential for neurogenesis but not gliogenesis in fetal neural stem cells. Dev Growth Differ, 2024.
Herrmann, A., A.K. Meyer, L. Braunschweig, L. Wagenfuehr, F. Markert, D. Kolitsch, V. Vukicevic, C. Hartmann, M. Siebert, M. Ehrhart-Bornstein, A. Hermann, and A. Storch, Notch is Not Involved in Physioxia-Mediated Stem Cell Maintenance in Midbrain Neural Stem Cells. Int J Stem Cells, 2023.
Helf, C., M. Kober, F. Markert, J. Lanto, L. Overhoff, K. Badstubner-Meeske, A. Storch, and M. Fauser, Subthalamic nucleus deep brain stimulation induces nigrostriatal dopaminergic plasticity in a stable rat model of Parkinson's disease. Neuroreport, 2023.
Markert, F. and A. Storch, Hyperoxygenation During Mid-Neurogenesis Accelerates Cortical Development in the Fetal Mouse Brain. Front Cell Dev Biol, 2022.
Braunschweig, L., J. Lanto, A.K. Meyer, F. Markert, and A. Storch, Serial Gene Expression Profiling of Neural Stem Cells Shows Transcriptome Switch by Long-Term Physioxia from Metabolic Adaption to Cell Signaling Profile. Stem Cells Int, 2022.
Fauser, M., M. Ricken, F. Markert, N. Weis, O. Schmitt, J. Gimsa, C. Winter, K. Badstubner-Meeske, and A. Storch, Subthalamic nucleus deep brain stimulation induces sustained neurorestoration in the mesolimbic dopaminergic system in a Parkinson's disease model. Neurobiol Dis, 2021.
Markert, F., L. Muller, K. Badstubner-Meeske, and A. Storch, Early Chronic Intermittent Maternal Hyperoxygenation Impairs Cortical Development by Inhibition of Pax6-Positive Apical Progenitor Cell Proliferation. J Neuropathol Exp Neurol, 2020.
Fauser, M., G. Weselek, C. Hauptmann, F. Markert, M. Gerlach, A. Hermann, and A. Storch, Catecholaminergic Innervation of Periventricular Neurogenic Regions of the Developing Mouse Brain. Front Neuroanat, 2020.
Wagenfuhr, L., A.K. Meyer, L. Marrone, and A. Storch, Oxygen Tension Within the Neurogenic Niche Regulates Dopaminergic Neurogenesis in the Developing Midbrain. Stem Cells Dev, 2016.
Braunschweig, L., A.K. Meyer, L. Wagenfuhr, and A. Storch, Oxygen regulates proliferation of neural stem cells through Wnt/beta-catenin signalling. Mol Cell Neurosci, 2015.
Wagenfuhr, L., A.K. Meyer, L. Braunschweig, L. Marrone, and A. Storch, Brain oxygen tension controls the expansion of outer subventricular zone-like basal progenitors in the developing mouse brain. Development, 2015.
Equipment (a selection)
![[Translate to English:] PCR](/fileadmin/_processed_/5/1/csm_PCR_395993ee61.jpg)
- high-resolution fluorescence microscope AxioObserver.Z1 with Apotome (Zeiss)
- Oxygen chamber/Glove-Box type ITA950 (InerTec) *
- Incubators with oxygen control CB160 (Binder)
- Research Cryostat /Cryomicrotome CM3050S (Leica) *
- real-time PCR device Rotor gene (Qiagen)
* co-financed by the European Union from the European Regional Development Fund
Present members
Prof. Dr. Alexander Storch
Group Leader
Dr. Franz Markert
PostDoc
M.Sc. Jennifer Lanto
PhD Student
M.Sc. Svenja Koch
PhD Student
M.Sc. Lisa Große
Research Assisstant
Annika Mund
MD Student
Jürke Hartz
MD Student
Theresa Hippelein
MD Student
Past members
Andreas Madlener
MD Student
Julia Neitzel
MD Student
Kashma Salih
MD Student
Monika Vehlken
MD Student
Tim Theege
MD Student
Laura Pohl
Bachelor Thesis
Luisa Müller
Bachelor Thesis

![[Translate to English:] Storch](/fileadmin/_processed_/3/d/csm_Storch_f7152f16b7.jpg)
![[Translate to English:] MV](/fileadmin/_processed_/9/b/csm_MV_06acda98c7.jpg)
![[Translate to English:] EU](/fileadmin/_processed_/8/f/csm_EU_ef61ab803c.jpg)